 Free |
|
Alcoholic fermentation by yeast cells
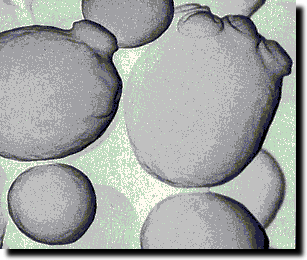 In
brewing, alcoholic fermentation is the conversion of sugar into carbon
dioxide gas (CO2) and ethyl alcohol. This process is carried out by yeast
cells using a range of
enzymes. This is in fact a complex series of conversions that brings about
the conversion of sugar to CO2 and alcohol. Yeast is a member of the fungi
family which I like to think of as plants but strictly they are neither
plant nor animal. To be specific yeast is a eukaryotic micro-organism. Not
all yeasts are suitable for brewing. In brewing we use the sugar fungi form of yeast. These
yeast cells gain
energy from the conversion of the sugar into carbon dioxide and alcohol. The
carbon dioxide by-product bubbles
through the liquid and dissipates into the air. In confined spaces the
carbon dioxide dissolve in the liquid making it fizzy. The pressure build up
caused by C02 production in a confined space can be immense. Certainly enough to cause the explosion of a sealed
glass bottle. Alcohol is the other by-product of fermentation. Alcohol remains in the liquid which is great for
making an alcoholic beverage but not for the yeast cells,
as the yeast dies when the alcohol exceeds its tolerance level.
In
brewing, alcoholic fermentation is the conversion of sugar into carbon
dioxide gas (CO2) and ethyl alcohol. This process is carried out by yeast
cells using a range of
enzymes. This is in fact a complex series of conversions that brings about
the conversion of sugar to CO2 and alcohol. Yeast is a member of the fungi
family which I like to think of as plants but strictly they are neither
plant nor animal. To be specific yeast is a eukaryotic micro-organism. Not
all yeasts are suitable for brewing. In brewing we use the sugar fungi form of yeast. These
yeast cells gain
energy from the conversion of the sugar into carbon dioxide and alcohol. The
carbon dioxide by-product bubbles
through the liquid and dissipates into the air. In confined spaces the
carbon dioxide dissolve in the liquid making it fizzy. The pressure build up
caused by C02 production in a confined space can be immense. Certainly enough to cause the explosion of a sealed
glass bottle. Alcohol is the other by-product of fermentation. Alcohol remains in the liquid which is great for
making an alcoholic beverage but not for the yeast cells,
as the yeast dies when the alcohol exceeds its tolerance level.
Overall chemistry of fermentation

The overall process of fermentation is to convert glucose sugar (C6H12O6) to alcohol (CH3CH2OH) and carbon dioxide gas (CO2). The reactions within the yeast cell which make this happen are very complex but the overall process is as follows:
C6H12O6
====> 2(CH3CH2OH)
+ 2(CO2)
+ Energy (which is stored in ATP)
Sugar ====>
Alcohol
+ Carbon dioxide gas
+ Energy
(Glucose)
(Ethyl alcohol)
From the above it seems nice an simple chemistry one mole of glucose is converted into two moles of ethanol and two moles of carbon dioxide but in reality it is far from this clear. There are many by products. In addition to CO2 and alcohol, the sugar is incorporated into other by products such as yeast biomass, acids (pyruvic, acetaldehyde, ketoglutaric, lactic), glycerol. Hence if you read many home brewing books there is a table estimating the conversion of sugar into alcohol. These tables tend to be derived from measurements rather than a set formula. The efficiency of the yeast and fermentation conditions alters the proportions of various by-products meaning a simple single formula is not available. Wine makers will see different efficiencies to beer makers. Fermentation conditions such as temperature vary the production of by products. This knowledge is used by wine makers to get fuller bodied wines by brewing in conditions that causes fermentation to produce more of the by-product glycerol.
Fermentation by-product Glycerol gives wine its body. From time to time you read in the press a very shocking tale of people adding anti-freeze to wine but this statement on its own does not tell you the full extent of the danger. Bear in mind Glycerol can be used as an anti-freeze and is a natural by product of fermentation but not all anti-freeze use glycerol, most use very toxic alternatives. So the statement "Anti-freeze added to wine" does not tell you if highly toxic chemicals were added or just Glycerol to supplementing the natural Glycerol content. In fact Glycerol is used in health foods and is essential in fine wines. Wine produced in conditions where there was low production of the glycerol by-product can tempt the producer to add something to boost the wine's body. Adding food grade Glycerol to boost a wine's body is not ideal but no need for panic as glycerol is natural and is often used in food products. Adding toxic anti-freeze to boost a wine's body can and does kill people.
Note: The sugars used can be a range of fermentable
sugars. These sugars are converted by enzymes to glucose which is then
converted to alcohol and CO2. Some sugars are not able to be
fermented and will remain in the liquid.
Fermentation and yeast in brewing
Brewer's yeast tolerate up to about 5% alcohol. Beyond this alcohol level the yeast cannot continue fermentation. Wine yeast on the other hand tolerates up to about 12% alcohol. The level of alcohol tolerance by yeast varies from 5% to about 21% depending on yeast strain and environmental conditions.
The fermentation process has limits such as temperature. Greater than 27C kills the yeast less and than 15C results in yeast activity which is too slow.
Not all sugars are fermentable. Non fermentable sugars in solution will remain after fermentation and will result in a sweeter end product. Malt has non fermentable sugars which can be used to balance the bitterness of the hops. The amount of sugar in the solution can be too much and this can prevent fermentation. Some wine recipes suggest adding the sugar in parts throughout fermentation rather that all at the beginning. This is especially true if the brew is aimed at producing a high level of alcohol. Some yeast strains have evolved to handle higher sugar levels. Yeast such as Tokay and Sauterne handle high levels of sugar. The normal, home brewing, fermentation is in two parts.
Part 1
Aerobic (Oxygen is present)
This is the initial rapid process where the yeast is doubling its colony size every four hours.
(Usually 24-48 hours)Part 2
Anaerobic. (No oxygen present)
Slower activity and the yeast focuses on converting sugar to alcohol rather that increasing the number of yeast cells.
(This process can take from days to weeks depending on the yeast and the recipe)
Why does yeast stop working at certain levels of alcohol?
The ability of yeast cells to convert sugar into Carbon dioxide and Alcohol is down to enzymes. Several enzymes are involved each does its step in the process. The final step is Zymase reduction which takes the end product of the other enzymes (acetaldehyde/glycerol), and turns this into good old ethyl alcohol. Sadly high concentrations alcohol actually destroys enzymes and kills the yeast cell. Different strains of yeast can tolerate different concentrations of alcohol.. Brewers yeast cannot withstand much beyond 5 or 6% Alcohol by volume. Wine yeast is more tolerant at a range of 10-15%
Specially cultured strains of yeast with the correct environment can withstand alcohol levels up to 21% alcohol.
Our NEW! LinkedIn group Fermentation Specialists
Specifically for Fermentation professionals and students. A place to share knowledge, ask questions and get involved in discussions.
Jobs and Product Promotion tabs for you to use. Be an early joiner and help make this group world class.
[Home] [Sitemap] [Fermentation] [Cider] [Beer] [Wine] [Other drinks] [Shops] [Recipe Design] [Grow your own] [Reviews] [Hangover]
FREE home brewing magazines

CONTACT: Email: Do drop us a line.














 All text on this site is purely the various contributing author's personal views and
should not be taken as fact.
All text on this site is purely the various contributing author's personal views and
should not be taken as fact.